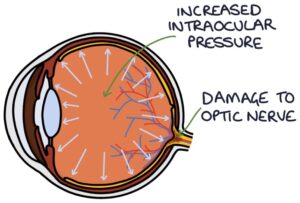
Glaucoma refers to the optic nerve damage caused by a rise in intraocular pressure. Raised intraocular pressure is caused by a blockage in aqueous humour trying to escape the eye. There are two types of glaucoma:
- Open-angle glaucoma
- Acute angle-closure glaucoma
Basic Anatomy and Physiology
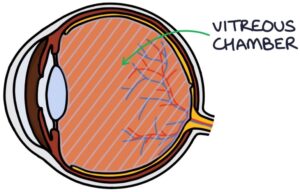
The vitreous chamber of the eye is filled with vitreous humour.
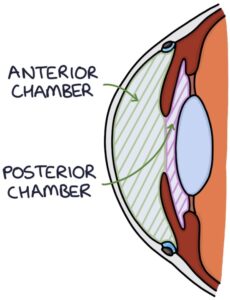
The anterior chamber (between the cornea and iris) and the posterior chamber (between the lens and iris) are filled with aqueous humour.
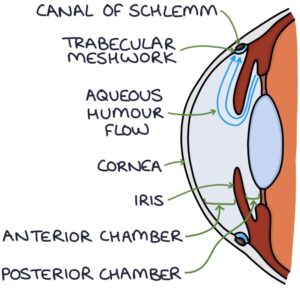
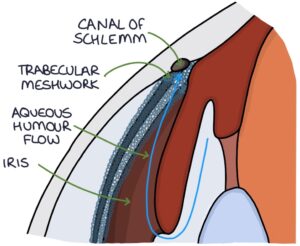
The aqueous humour supplies nutrients to the cornea. It is produced by the ciliary body. It flows through the posterior chamber and around the iris to the anterior chamber. It drains through the trabecular meshwork to the canal of Schlemm at the angle between the cornea and the iris. From the canal of Schlemm, it eventually enters the general circulation.
Normal intraocular pressure is 10-21 mmHg, created by the resistance to flow through the trabecular meshwork.
Pathophysiology
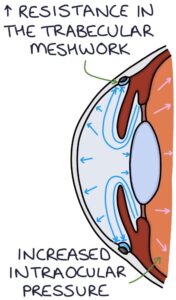
With open-angle glaucoma, there is a gradual increase in resistance to flow through the trabecular meshwork. The pressure slowly builds within the eye.
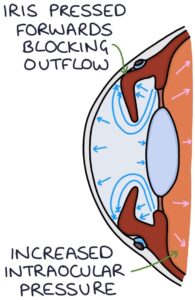
With acute angle-closure glaucoma, the iris bulges forward and seals off the trabecular meshwork from the anterior chamber, preventing aqueous humour from draining. There is a continual build-up of pressure and an acute onset of symptoms. Acute angle-closure glaucoma is an ophthalmological emergency.
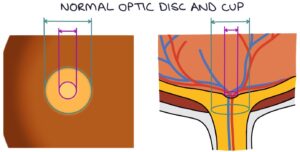
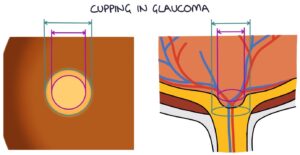
Raised intraocular pressure causes cupping of the optic disc. In the centre of the optic disc is an indent called the optic cup. The optic cup usually is less than 50% of the size of the optic disc. Raised intraocular pressure causes this indent to become wider and deeper, described as “cupping”. A cup-disk ratio greater than 0.5 is abnormal.
Risk Factors
Risk factors for open-angle glaucoma include:
- Increasing age
- Family history
- Black ethnic origin
- Myopia (nearsightedness)
Presentation
The rise in intraocular pressure may be asymptomatic for a long time and diagnosed by routine eye testing.
Glaucoma affects the peripheral vision first, resulting in a gradual onset of peripheral vision loss (tunnel vision). It can also cause:
- Fluctuating pain
- Headaches
- Blurred vision
- Halos around lights, particularly at night
Measuring Intraocular Pressure
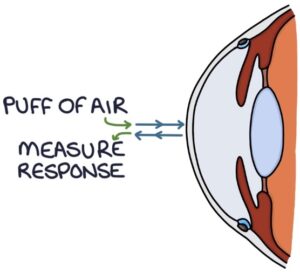
Non-contact tonometry is the commonly used machine for estimating intraocular pressure by opticians. It involves shooting a “puff of air” at the cornea and measuring the corneal response. It is less accurate but gives an estimate for general screening purposes.
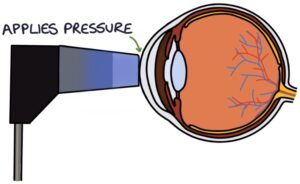
Goldmann applanation tonometry is the gold-standard way to measure intraocular pressure. It involves a device mounted on a slip lamp that makes contact with the cornea and applies various pressures.
Diagnosis
Diagnosis is based on:
- Goldmann applanation tonometry for the intraocular pressure
- Slit lamp assessment for the cup-disk ratio and optic nerve health
- Visual field assessment for peripheral vision loss
- Gonioscopy to assess the angle between the iris and cornea
- Central corneal thickness assessment
Management
Treatment is typically started at an intraocular pressure of 24 mmHg or above.
360° selective laser trabeculoplasty is recommended in the NICE guidelines (updated 2022) for all patients needing treatment. During the procedure, a laser is directed at the trabecular meshwork, improving drainage. It may delay or prevent the need for eye drops. A second procedure may be necessary at a later date.
Prostaglandin analogue eye drops (e.g., latanoprost) are the first-line medical treatment. They increase uveoscleral outflow. Notable side effects are eyelash growth, eyelid pigmentation and iris pigmentation (browning).
Other eye drop options (as eye drops) include:
- Beta-blockers (e.g., timolol) reduce the production of aqueous humour
- Carbonic anhydrase inhibitors (e.g., dorzolamide) reduce the production of aqueous humour
- Sympathomimetics (e.g., brimonidine) reduce the production of aqueous fluid and increase the uveoscleral outflow
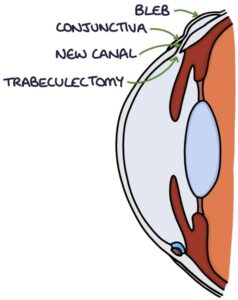
Trabeculectomy surgery may be required where other treatments are ineffective. This involves creating a new channel from the anterior chamber through the sclera to a location under the conjunctiva, causing a bleb on the conjunctiva. From here, it is reabsorbed into the general circulation.


.png)